Our EU Member States Share Rapid Antigen Test Performance Data Ideas

The Ultimate Guide To MSDS for SARS-Cov-2 antigen test.pdf - BioVendor
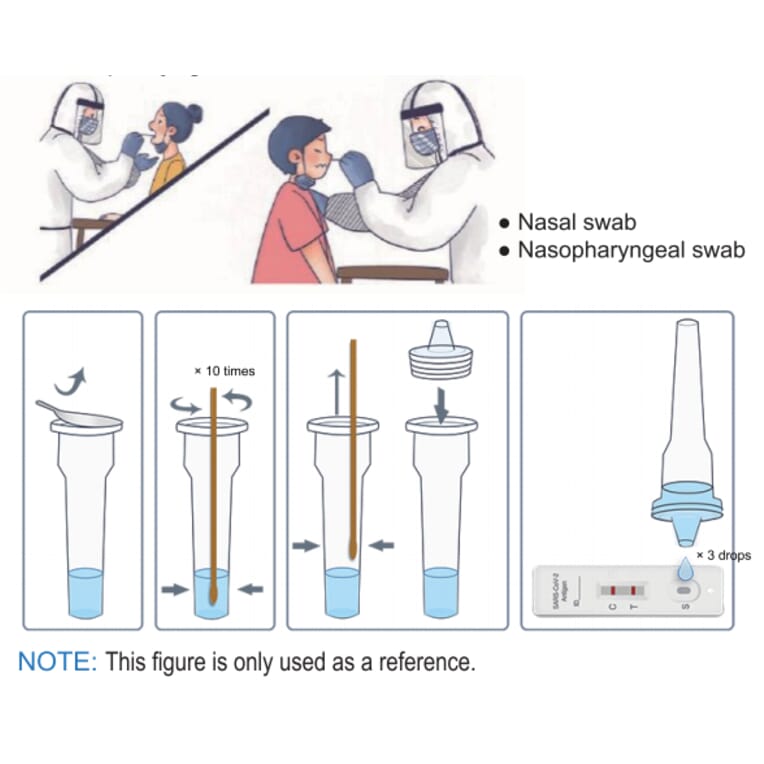
Throughout tasting, the swab head must be totally placed into the nasal cavity and carefully turned 5times. After elimination, the swab head must be tested in the other nasal cavity in the exact same way to ensure that sufficient samples are taken. Prior to the test, the double-sided adhesive protective layer ought to be removed beforehand to prevent liquid splashing.
Thread the swab sample through the bottom of well B into well A. Include 6 drops of the diluent into well A. Do not drop the diluent into the other wells. Rotate the shaft, two rounds each direction. During the test, the test card need to be put on the horizontal desktop.
After covering the left side, gently press the adhesive position to make the two sides entirely fit and start timing. Wait till the purple band appears. The test outcome need to read within 15-20 minutes.
The 10-Second Trick For Colloidal Gold Immunochromatography Nasal Swab Saliva
The novel corona infections belong to the genus. SARSCo, V-2 is an intense respiratory infectious disease. Individuals are usually susceptible. Currently, the clients infected by the novel corona infection are the main source of infection; asymptomatic contaminated individuals can also be an infectious source. Based on the present epidemiological examination, the incubation duration is 1 to 2 week, mainly 3 to 7 days.
Nasal congestion, runny nose, aching throat, myalgia and diarrhoea are found in a few cases. Antigen is generally detectable in upper breathing specimens throughout the severe phase of infection. Quick medical diagnosis of SARS-Co, V-2 infection will help health care professionals to treat clients and control the disease more effectively and efficiently.
BIOHIT Healthcare (Hefei) Co., Ltd.
The declaration type can be used for research study and approval type functions only.
SARS-CoV-2 Rapid IgM/IgG Antibody Test Kit (Colloidal Gold Method) - ADS Biotec
Coronavirus COVID-19 Antigen Rapid Test - Aurora Biomed
The Main Principles Of SARS-CoV-2 Antigen Rapid Detection kit - 中科先见
SARS-COV-2 Antigen Rapid Test Package (Swab) is a solid phase immunochromatographic assay for the fast, qualitative and differential detection of antigen to 2019 Novel Coronavirusi in human swab. This test supplies only a preliminary test result. For that reason, any reactive specimen with the SARS-COV-2 Antigen Quick Test package (Swab) should be verified with alternative testing approach(s) and medical findings.
(Colloidal Gold Immunochromatography)Extreme severe respiratory syndrome coronavirus 2 (SARS-Co, V-2, or 2019-n, Co, V) is an enveloped non-segmented positive-sense RNA virus. It is the reason for coronavirus illness 2019 (COVID-19), which is infectious in human beings. This Piece Covers It Well -Co, V-2 has several structural proteins consisting of spike (S ), envelop( E ), membrane( M) and nucleocapsid (N).
